| Bactiguard AB |
| Registration Date | 1 Jul 2018 |
| Revision Date | 1 Jul 2018 |
| Share |
Medicine Medical Supplies
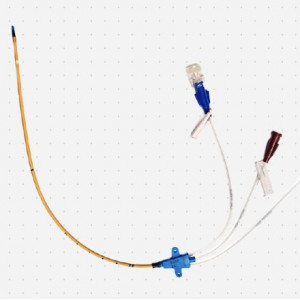
Tracheal tubegold-palladium-silver
Au-Pd-Ag Ultra-thin FilmSAVES LIVES REDUCES RESPIRATORY TRACT INFECTIONS REDUCES THE USE OF ANTIBIOTICS REDUCES HEALTHCARE COSTS
The Bactiguard Infection Protection Endotracheal Tubes (BIP ETT and BIP ETT Evac), with the Bactiguard coating, reduces the risk of microbial adhesion, colonization and subsequent respiratory infections. It is available both with and without subglottic secretion drainage (SSD), a mechanical feature in itself proven to reduce the risk of ventilator associated pneumonia (VAP). Bactiguard coated ETTs have been shown to effectively reduce microbial adhesion to the device by up to 98% in microbiological in vitro studies with relevant clinical isolate strains3. In addition, a clinical study on 100 patients comparing a standard uncoated endotracheal tube with BIP ETT concluded that the Bactiguard coating reduced the incidence of VAP by 67%4. The combination of subglottic secretion drainage and the unique Bactiguard coating on the new tube, BIP ETT Evac, is designed to offer the best protection against VAP available on the market.
The BIP ETT is intended for use in airway management by oral or nasal intubation of the trachea. It is used to secure an open airway during anesthesia or when intubation is necessary as part of standard medical care. The beveled tip, Murphy Eye and high volume-low pressure cuff are designed to minimize the risk of damages to the patients’ trachea and ensure safe usage. The catheter is made of PVC and coated with the Bactiguard coating on both the inside and outside of the tube, and can be used up to 30 days.
In a clinical study on 100 patients comparing a standard uncoated endotracheal tube with the BIP ETT, it was concluded that the coating reduced the incidence of ventilator associated pneumonia by 67% (OR 3.42; p=0.14)4. This prospective, randomized and independent investigation included toxicological intensive care patients suffering from poisoning. In addition to the infection rate, length of hospital stay as well as antibiotic use was also monitored. The BIP ETT group used significantly less antibiotics (OR 0.3, p=0.05) and stayed in hospital 2 days less in average (18 and 16 days, respectively).
The Bactiguard solution is unique, tissue friendly and safe for patient use. As opposed to other coating technologies, which depend on the release of substances, which kill bacteria, e.g. large amounts of silver ions, chlorhexidine or antibiotics, the Bactiguard coating is neither toxic nor pharmacologic.12 The Bactiguard coated BIP ETTs have a strong biocompatibility profile.