| Medicago Inc. |
| No Title | 0.16 MB |
| Registration Date | 11 May 2020 |
| Revision Date | 11 May 2020 |
| Share |
Medicine Pharmaceuticals
COVID-19 vaccine
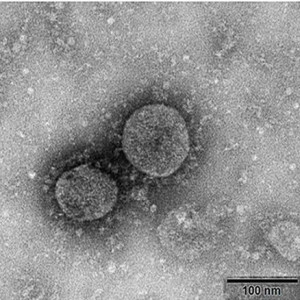
Medicago has announced that they have successfully produced a Virus-Like Particle (VLP) of the coronavirus just 20 days after obtaining the SARS-CoV-2 (virus causing the COVID-19 disease) gene. Production of the VLP is the first step in developing a vaccine for COVID-19 which will now undergo preclinical testing for safety and efficacy. Once this is completed, Medicago expects to discuss with the appropriate Health Agencies to initiate human trials of the vaccine by summer (July/August) 2020. Medicago is also using its technology platform to develop antibodies against SARS-CoV-2 in collaboration with the Laval University’s Infectious Disease Research Centre headed. These SARS-CoV-2 antibodies could potentially be used to treat people infected by the virus. This research is being funded, in part, by the Canadian Institutes for Health Research (CIHR). Medicago is a leader in plant-based technology having previously demonstrated its capability to be a first
responder in a flu pandemic. In 2009, the company produced a research-grade vaccine candidate against H1N1 in just 19 days. In 2012, Medicago manufactured 10 million doses of a monovalent influenza vaccine within one month for the Defense Advanced Research Projects Agency (DARPA), part of the U.S. Department of Defense. In 2015, Medicago also demonstrated that it could rapidly produce an antiEbola monoclonal antibody cocktail for the Biomedical Advanced Research and Development Authority (BARDA), part of the U.S. Department of Health and Human Services. The pace of their initial progress in COVID-19 is attributable to the capability of our plant-based platform
which is able to produce vaccine and antibody solutions to counteract this global public health threat. The ability to produce a candidate vaccine within 20 days after obtaining the gene is a critical differentiator for our proven technology. This technology enables scale-up at unprecedented speed to potentially combat COVID-19.
The collaborative efforts established between the research team at Laval University and Medicago have been very successful in developing
unique antibodies against infectious diseases such as RSV and HMPV and that experience gives them confidence for successful identification of therapeutic antibodies against SARS-CoV-2. Medicago’s first product, a seasonal recombinant quadrivalent VLP vaccine for active immunization against influenza, is currently under review by Health Canada following the completion of a robust safety and efficacy clinical program involving over 25,000 patients.
In the preclinical evaluation/regulatory stage