| Virpax® Pharmaceuticals |
| Registration Date | 18 Jan 2023 |
| Revision Date | 18 Jan 2023 |
| Share |
Medicine Pharmaceuticals
Epilepsy Drug
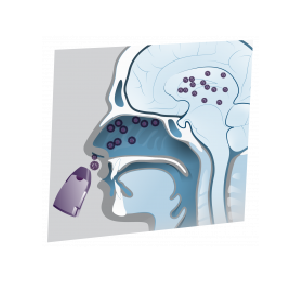
NobrXiol is a patented pharmaceutical-grade cannabidiol intranasal spray powder being developed to manage Rare Pediatric Epilepsy (Lennox-Gastaut Syndrome (LGS) and Dravet Syndrome).
NobrXiol may be using significantly less pharmaceutical-grade CBD than current FDA approved oral CBD dosing
NobrXiol may achieve higher efficiency via the nasal route and reduce peripheral side effects like dose dumping due to high-fat meals
Since peripheral exposure via the plasma will be reduced by the nose to brain delivery, we believe there will be negligible liver first-pass metabolism
NobrXiol may not be metabolized in the liver avoiding drug to drug interactions caused by oral pharmaceutical-grade CBD
By avoiding the first pass effect Intranasal NobrXiol may eliminate enzymatic degradation or deactivation. Enzymatic deactivation occurs when an enzyme changes the structure of a neurotransmitter so that the receptor no longer recognizes the neurotransmitter